Multivariate Continuous Bayesian Clinical Trials in R Pdf
- Original Research
- Published:
Bayesian Design for Pediatric Clinical Trials with Binary Endpoints When Borrowing Historical Information of Treatment Effect
Therapeutic Innovation & Regulatory Science volume 55,pages 360–369 (2021)Cite this article
Abstract
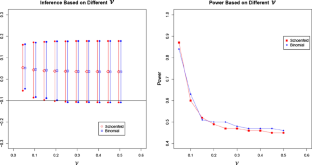
The efficacy evaluation in pediatric population is an important component of drug development and is generally required by the regulatory agencies. It is often challenging to enroll pediatric subjects for a large trial especially when the incidence rate is low in certain disease areas. Bayesian framework can provide analytic avenues to effectively utilize historical information of the treatment effect and help make pediatric trials more efficient by reducing the sample size when there is evidence to suggest similarity of the treatment responses between the populations. Schoenfeld et al. (Clin Trials 6(4):297–304, 2009) proposed a Bayesian hierarchical model for efficacy extrapolation for continuous endpoints, which connects a single historical trial and the current trial by a variance parameter in the prior distribution. In this manuscript, we extend the existing model to borrow strength from multiple historical trials under the same assumptions and develop a quantitative method to borrow historical information more efficiently. Furthermore, we extend Schoenfeld's method based on continuous endpoints to binary endpoints with a hierarchical binomial model to extrapolate efficacy. Sensitivity analyses for the underlying assumptions are discussed with simulations and the methods are illustrated with a real case study, along with some practical considerations about how to choose the prior distribution.
Access options
Buy single article
Instant access to the full article PDF.
39,95 €
Price includes VAT (Indonesia)
References
-
Food US, Administration Drug. Exposure-response relationships—study design, data analysis, and regulatory applications. 2018.
-
Mulugeta Y, Barrett JS, Nelson R, Eshete AT, Mushtaq A, Yao L, Glasgow N, Mulberg AE, Gonzalez D, Green D, Florian J. Exposure matching for extrapolation of efficacy in pediatric drug development. J Clin Pharmacol. 2016;56(11):1326–34.
-
Dixon DO, SimonSimon R. Bayesian subset analysis. Biometrics. 1991;47:871–81.
-
Spiegelhalter JD, Abrams RK, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation, vol. 13. Hoboken: Wiley; 2004.
-
Spiegelhalter David J, et al. Incorporating Bayesian ideas into health-care evaluation. Stat Sci. 2004;19(1):156–74.
-
GamaloSiebers M, Savic J, Basu C, Zhao X, Gopalakrishnan M, Gao A, Song G, Baygani S, Thompson L, Xia HA, Price K. Statistical modeling for bayesian extrapolation of adult clinical trial information in pediatric drug evaluation. Pharm Stat. 2017;16(4):232–49.
-
Kaur A, Skoner D, Ibrahim J, Li Q, Lockey RF, Blaiss M, Bufe A, Andersen JS, Canonica GW, Nolte H. Effect of grass sublingual tablet immunotherapy is similar in children and adults: a bayesian approach to design pediatric sublingual immunotherapy trials. J Allergy Clin Immunol. 2018;141(5):1744–9.
-
Schoenfeld DA, Zheng H, Finkelstein DM. Bayesian design using adult data to augment pediatric trials. Clin Trials. 2009;6(4):297–304.
-
Heinz S, Sandro G, Satrajit R, Anthony OH, David S, Beat N. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics. 2014;70(4):1023–32.
-
Food US, Administration Drug. Guidance for the use of bayesian statistics in medical device clinical trials. Guidance for industry and FDA staff. US FDA Docket. 2010;(2006D-0191):50.
-
Rubin DB. Bayesianly justifiable and relevant frequency calculations for the applies statistician. Ann Stat. 1984;12:1151–72.
-
Box George EP. Sampling and bayes' inference in scientific modelling and robustness. J R Stat Soc Series A (General). 1980;143(4):383–404.
-
Grieve AP. Idle thoughts of a well-calibrated Bayesian in clinical drug development. Pharm Stat. 2016;15(2):96–108.
-
Smania G, Baiardi P, Ceci A, Cella M, Magni P. Model-based assessment of alternative study designs in pediatric trials part ii: Bayesian approaches. CPT Pharmacometr Syst Pharmacol. 2016;5(8):402–10.
-
Viele K, Berry S, Neuenschwander B, Amzal B, Chen F, Enas N, Hobbs B, Ibrahim JG, Kinnersley N, Lindborg S, Micallef S. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014;13(1):41–54.
-
Morris Tim P, White Ian R, Crowther MJ. Using simulation studies to evaluate statistical methods. Stat Med. 2019;38(11):2074–102.
-
Salman FT, DiCristina C, Chain A, Afzal AS. Pharmacokinetics and pharmacodynamics of aprepitant for the prevention of postoperative nausea and vomiting in pediatric subjects. J Pediatr Surg. 2019;54(7):1384–90.
-
Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, Pueyo J, Chelly JE, Carides AD, Reiss T, Evans JK. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase iii trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99(2):202–11.
-
Food US, Administration Drug. Interacting with the fda on complex innovative trial designs for drugs and biological products. US Department of Health and Human Services, Federal Registrar. https://www.fda.gov/media/130897/download. 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Jin, M., Li, Q. & Kaur, A. Bayesian Design for Pediatric Clinical Trials with Binary Endpoints When Borrowing Historical Information of Treatment Effect. Ther Innov Regul Sci 55, 360–369 (2021). https://doi.org/10.1007/s43441-020-00220-5
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1007/s43441-020-00220-5
Keywords
- Sample size calculations
- Bayesian framework
- Prior distributions
- Hierarchical model
- Pediatric clinical trials
Source: https://link.springer.com/article/10.1007/s43441-020-00220-5
0 Response to "Multivariate Continuous Bayesian Clinical Trials in R Pdf"
Post a Comment